Insights
Get expert insights into cough science, monitoring and research
Get expert insights into cough science, monitoring and research
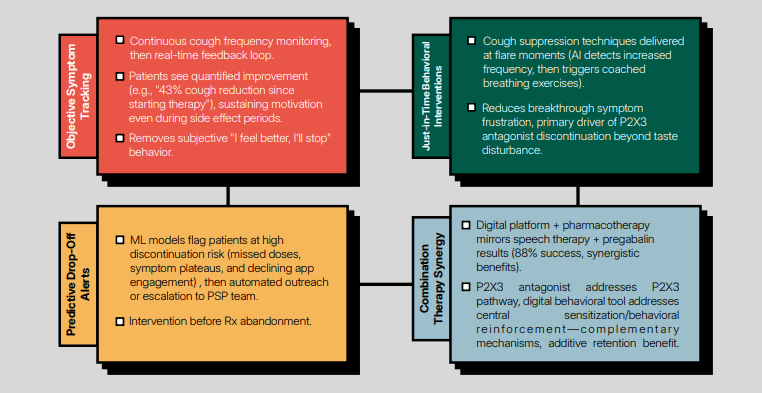














Cough monitoring remains a significant endpoint in clinical trials, especially in studies targeting respiratory diseases. While accurate objective cough counting is essential for evaluating drug efficacy and understanding disease progression, until recently there has been no reliable technology available to ensure that cough is accurately measured. Over the last decade, several technologies have emerged that are capable of performing reliable cough monitoring in the real world. However, there remains a gap in regulatory clearance for devices explicitly designed to count coughs.
Currently, several systems are in use in various trials and some of them have been validated in peer-reviewed studies. However, none have received FDA clearance specifically for cough counting.
That’s right: Despite the widespread use of cough-counting technologies, no system has yet received explicit FDA clearance for counting coughs.
When a medical device receives 510(k) clearance from the U.S. Food and Drug Administration (FDA), it means the device has been shown to be substantially equivalent to a legally marketed device, known as a “predicate.” Essentially, the manufacturer must demonstrate that their product is as safe and effective as an existing, similar device.
It’s important to note that 510(k) clearance differs from a de-novo FDA approval for novel solutions, such as cough counting devices or technologies, which have not yet been cleared by the FDA. In a 510(k) pathway, the manufacturer provides evidence that the device is sufficiently similar to one already on the market. For devices like the Strados Resp Biosensor or VitaloJAK, 510(k) clearance was granted for sound recording, but not specifically for cough counting, meaning their ability to count coughs has not been evaluated, validated or cleared by the FDA.
The Strados Resp Biosensor is a device that has capabilities to monitor respiratory sounds, including coughs. The device has received FDA clearance only to “acquire, record and store lung sounds”... “the device stores the data for later playback, review, and analysis by a clinician”. The validation carried out for the device’s 510(k) clearance was “performed to validate the quality and fidelity of the subject device’s recorded lung sounds”; the FDA has never cleared the accuracy of the Strados method for counting coughs. The FDA classifies the Strados device as a “Medical magnetic tape recorder”, not a cough counter.
There is a distinction between a "Medical magnetic tape recorder," and a “cough counter”. This distinction is very important for drug developers, researchers and clinicians relying on the device for cough monitoring in studies, as it means that, while the system’s ability to record sounds is verified by the regulator, the system’s cough counting capabilities have not been verified or cleared by the FDA.
Similar to the Strados Resp Biosensor, the VitaloJAK recording device has received FDA clearance, but only to “acquire, record and store” sound; the processes used by the VitaloJAK system to count coughs involves a combination of proprietary software compression algorithm and manual / human annotation of cough sounds. However, the FDA has not cleared the accuracy of the algorithm and manual / human annotation for cough counting, meaning that VitaloJAK is not FDA cleared for “cough monitoring”.
The validation process for VitaloJAK’s 510(k) clearance focused on the device’s performance in capturing audio, not on its ability to count coughs accurately. As with the Strados device, the FDA classifies VitaloJAK as a "Medical magnetic tape recorder," highlighting that its cough counting capabilities have not been endorsed. Furthermore, the FDA has explicitly emphasized that “this device and algorithm validation is not considered generalizable” and that “the VitaloJAK device holds an FDA 510(k) clearance as an audio recording device only. This does not include compression or cough counting”.
The Hyfe CoughMonitor Suite (CMS) is also not yet cleared by the FDA. Hyfe, the company building the Hyfe CoughMonitor Suite, is pursuing FDA de-novo clearance for its fully automated, AI-powered and validated, passive continuous cough monitoring system in adults with problematic cough of any etiology. However, though the technology is validated, it does not have FDA clearance for “cough counting”.
Several other cough counting devices, such as the Siva P3 and C-mo automated cough monitor, are currently in development or available on the market. However, none of these devices have received FDA clearance. While they may have been validated in specific studies or through peer-reviewed publications, like the previously mentioned systems, they lack FDA’s regulatory clearance.
To date, the FDA has not cleared any device, system, or software specifically for the purpose of counting coughs. The regulatory status of existing devices, such as the Strados Resp Biosensor and VitaloJAK, indicates that their clearance is for sound recording rather than cough counting. This regulatory gap presents challenges for drug developers, researchers, and clinicians who rely on precise cough data in clinical trials, potentially impacting the ability of the pharmaceutical industry to bring much-needed treatments to market.
As evidence supporting cough as a valuable biomarker grows and the demand for precise cough monitoring increases, there is a clear need for continued development and regulatory evaluation of these technologies. We anticipate that the FDA will eventually clear a device or a technology specifically for cough counting. In the meantime, drug developers, academics, clinicians and other users of existing systems should be mindful of the current limitations of the available technology: none have received FDA clearance specifically for cough counting.
When the FDA clears a medical device, it means the device has gone through extensive testing and evaluation to ensure it is safe and effective for its intended use. FDA clearance requires comprehensive clinical data, human factor studies and trials to prove that the device works as intended, provides a benefit to patients, and does not pose any unreasonable risks. Once a device receives FDA clearance, healthcare professionals and patients can have confidence that the device has been thoroughly vetted for safety and performance, according to its intended use case statement.
Researchers and clinical trial sponsors should be mindful of these limitations when designing studies that use cough frequency as an outcome measure. Moving forward, closer collaboration between technology developers and regulatory bodies will be crucial to aligning device capabilities with regulatory requirements, ensuring the availability of reliable tools for cough counting in clinical settings.
While FDA clearance is an important milestone for widespread clinical use, science need not wait for FDA clearance to drive meaningful progress. Many clinical trials are already underway utilizing cough monitoring devices classified as "nonsignificant risk devices," which only require approval from an Institutional Review Board (IRB). These devices, though not FDA-cleared for cough counting, have demonstrated their reliability and accuracy through rigorous scientific validation and real-world application. Researchers and drug developers can continue to push the boundaries of medical science, relying on tools that have been proven effective in studies and trials. By leveraging innovative technology - such as AI - the scientific community can continue advancing our understanding of respiratory conditions and accelerate the development of life-changing treatments without unnecessary delays.



