Insights
Get expert insights into cough science, monitoring and research
Get expert insights into cough science, monitoring and research














Dr. Henkle: My background is in infectious diseases and pulmonary infections, with a focus on epidemiology. I study these issues from a population perspective, which includes understanding the burden of disease, its epidemiology, and associated risk factors. Over time, I've also moved into clinical research, working on clinical trials to evaluate both new and existing interventions.
I joined Dr. Winthrop's team at OHSU in Portland over 10 years ago. One of the projects there was a population-based study aimed at understanding long-term outcomes in people with isolated NTM. This involved conducting phone interviews with about a hundred people in Oregon. During those interviews, I asked about symptoms and health-related quality of life. These conversations really opened my eyes to the patient experience, including the impact of cough on their quality of life. So, my interest in NTM has been long-standing, and my focus on understanding and measuring cough has evolved over time.
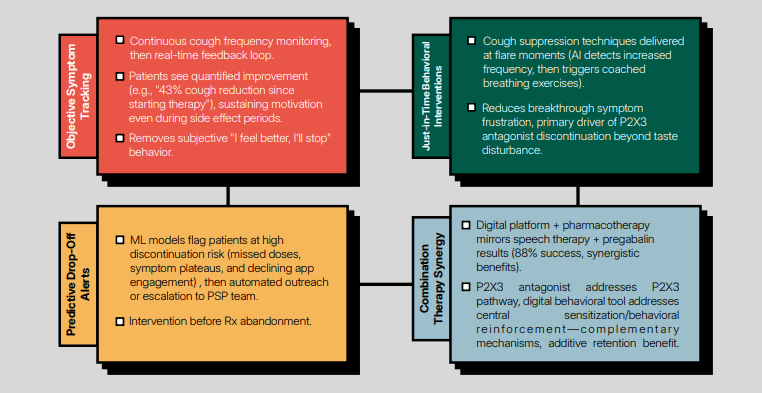
Dr. Henkle: Our current research focuses on patient-reported outcomes in individuals with NTM, and it's an observational cohort study, meaning there’s no intervention. We’re simply observing three different populations or cohorts that meet specific characteristics. This study builds on the work I started 10 years ago, where I interviewed patients trying to identify meaningful, patient-focused outcome measures, which is a top priority for NTM pulmonary disease.
Our main objectives are to better understand the trajectory of symptoms using patient-reported outcome measures, or PROs, to assess symptoms and health-related quality of life throughout the disease course. We’re also measuring self-reported toxicity and tolerability. Finally, we’ll correlate those symptoms with objective cough measurements using continuous cough monitoring.
Dr. Henkle: NTM is a chronic infection, often difficult to diagnose, and people can have it for a long time.
Our study includes three cohorts. The first is called NTM WATCH. This group consists of people who have initially isolated NTM, meaning they’ve had a positive bacterial culture, but it’s not yet clear if they meet the full case definition for the disease. We’re going to enroll them and follow their progress to see if they develop NTM disease, become culture-negative, or maintain a stable, mild infection. Essentially, we’re monitoring them while they're in this early stage of isolation. We'll also monitor cough in this group for 30 days to identify any patterns or indicators that might predict their outcomes.
The second cohort is NTM TREAT. This group includes patients who are starting treatment. Once NTM disease has progressed—whether due to symptoms or findings like worsening lung damage on CT scans—treatment is initiated. In these patients, we’ll measure symptoms, health-related quality of life, and cough.
The final cohort is NTM TRACK. These are people who have completed the treatment, which typically lasts 15 to 18 months. After completing treatment, there's a risk of recurrence, so we’ll follow this group to see if the disease comes back.
Dr. Henkle: We’ll be following participants for up to two years. This is because someone might initially be stable for a year but then start treatment later on. If that happens, we’ll move them into the NTM TREAT cohort to follow their treatment and any related patterns.
In the WATCH and TRACK cohorts, we’ll measure symptoms less frequently. However, once participants are in the TREAT cohort, we plan to monitor them more closely. This makes sense since they’ll be seeing their doctors more often during treatment anyway.
For cough monitoring, participants in both the WATCH and TREAT cohorts will wear a monitor. In the WATCH cohort, we’ll do a 30-day cough monitoring early on to see if cough characteristics can predict who might progress to meet the disease criteria and who might remain stable. For those in TREAT, we’ll monitor cough for 90 days to assess the impact of treatment. We have data suggesting that symptoms often improve within 90 days by self-report, so it would be great to also observe changes in their cough patterns during this time.
Dr. Henkle: We’re using PROs with a seven-day recall period. However, we don’t administer them every single week. The approach is more intensive at the beginning. For our TREAT patients, we collect PROs every week for the first two or three months, and then it becomes less frequent over time. The idea is to have intensive monitoring during the period when we expect the most significant changes, and then continue to track progress as time goes on.
Dr. Henkle: At this point, the study is still fairly descriptive, so we consider it more of a natural history study. We're focused on describing these longitudinal changes over time. However, we do plan to look at predictors of disease progression in the NTM WATCH cohort, treatment response in NTM TREAT, and disease recurrence in NTM TRACK.
Broadly speaking, if we were to hypothesize, we would expect that worsening symptoms and increased cough would be associated with poorer outcomes. What I find particularly interesting is understanding how closely the objective cough measurements correlate with patient self-reports. This will help us determine whether we need to track both measures to fully understand what's happening with the disease, or if one measure might be sufficient on its own. If there’s a strong correlation between the two, then we might not need to measure both.
Additionally, we may observe variability between patients, where the correlation between objective measures and self-reports is strong in some patients but weaker in others. If that’s the case, it will be important to understand why these differences exist, as they could provide valuable insights into the disease and its progression.
Dr. Henkle: From my perspective, always the patients—especially those who are newly diagnosed with NTM and are uncertain about what lies ahead. Our goal isn’t just to collect data for the sake of it, but to truly understand what’s useful to monitor, what can predict or expedite diagnosis, and when interventions are necessary. When patients are deciding whether to start treatment, they need to weigh the risks and benefits. The more information we have about treatments and their responses, the better-informed those decisions can be. We're particularly excited about this patient-focused research and how it can hopefully translate into more patient-centered care.
The second group that stands to benefit is busy clinicians. We hope that our research will help identify which changes in patients are clinically significant, making it easier for them to provide effective care.
I’m really excited about the potential to take complex data and distill it into something valuable. I’m a data person, so I enjoy working with numbers, graphs, and visuals. There’s still a lot we don’t know about cough patterns—things like frequency, severity, and quality. Understanding these aspects more thoroughly could be groundbreaking.
From a patient-reported perspective, I’m eager to see the development of a simple, easy-to-administer PRO that measures cough effectively. I’m interested in capturing a broad range of respiratory symptoms and health-related quality of life. Ideally, we’d create a comprehensive tool that can be used for care management and patient support.
Additionally, having a user-friendly interface where patients can access their own data is important. While some patients may not want to focus too much on daily details, others find it helpful. It’s valuable to be able to review trends and changes, like a spike in symptoms a month ago, and discuss these observations with their doctor. Presenting this information in a way that’s easy to understand could be very beneficial for both patients and clinicians.



